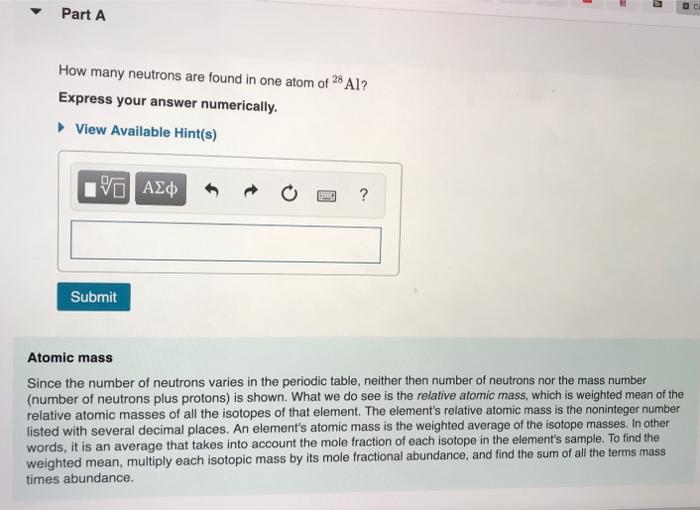
How Many Neutrons Are Found in One Atom of 28al
The number of neutrons N in an isotope of an element is the difference between the mass number and atomic number. 20 F has a mass number of fluorine.
Solved 3 Part A How Many Neutrons Are Found In One Atom Of Chegg Com
Set up the equation so that you end up with the UNIT you desire for an answer and the math will be correct.

. B Compounds are composed of atoms of more than one element. F B Na Question 2 Determine the number of electrons in the second shell n 2 of one Cl atom. How many protons are found in 1 atom of 28Al.
Neutrons Mass number - protons 20 - 9 11. It has an atomic number of 7 so it also has seven protons. Atomic number 13.
THIS IS THE BEST ANSWER Atomic mass 28Atomic number 13This impliesNumber of neutrons 28-13 15 HOPE IT HELPS Read More How many neutrons are found in one atom of 28al. The largest number of neutrons in any natural nuclide is 150 found in plutonium-244 but this nuclide only occurs in trace amounts on Earth. E The atoms of one element are the same as atoms of another element.
8 Question 3 Calculate the number of protons p electrons e and neutrons n0 in one atom of 29P. Neutrons protons and electrons. Silicon has three naturally occurring isotopes with the masses and natural abundances listed hereCalculate the atomic mass of silicon.
Mass number protons neutrons. Who are the experts. Number of Neutrons 65 - 30 35.
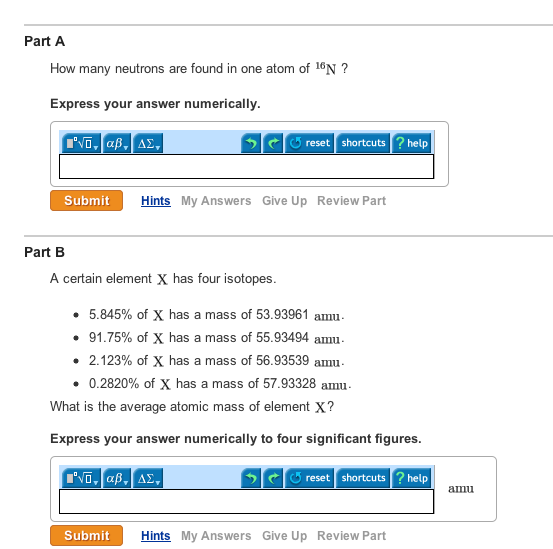
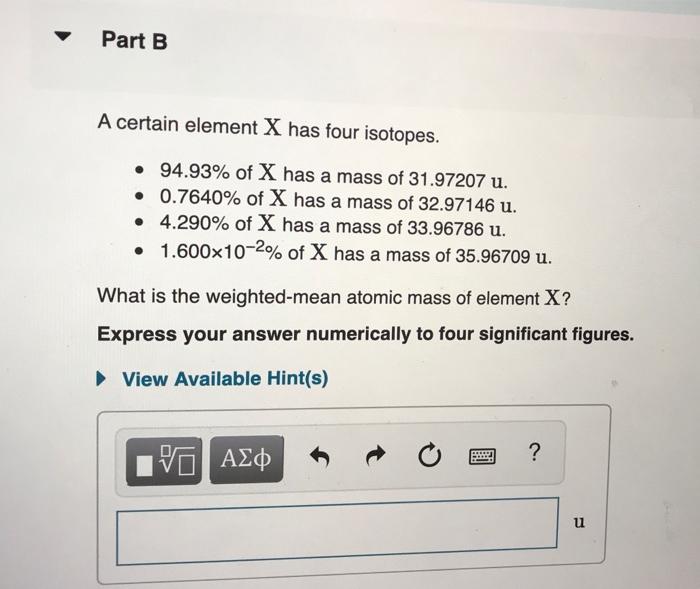
This chemistry video tutorial explains how to calculate the number of protons neutrons and electrons in an atom or in an ion. Rubidium has two naturally occurring isotopes with the masses and natural abundances listed hereCalculate the atomic mass of rubidium. 1600x10-2 of X has a mass of 3596709 amu.
How many neutrons are found in one atom of. 258 atoms 167 x 1026 amu These are just conversions. It also explains the differe.
The number of neutrons in an atom of 36Cl isotope is 19. Nitrogen is the seventh element on the periodic table. Click again to see term.
Aluminium-28 atom is the radioactive isotope of aluminium with relative atomic mass 27981910 and half-life of 225 min. 20 F has 11 neutrons. They have the same number of protons but different number of neutrons.
B Na F Correct Answer. Part A How many neutrons are found in one atom of 20F. These atoms are called isotopes.
An atom is made up of sub-atomic particles. Atom sub-atomic particles electrons protons neutrons Learn more about. For hydrogen 1008 is closer to 1 than 2 so lets call it 1.
In this case 36-1719. One atom of 20 F contains 11 neutrons. One neutral atom of nitrogen has seven protons seven neutrons and seven electrons.
Atomic mass 28. There are 13 protons in an atom of aluminum so the equation would be 27-1314. View Available Hints VAZO o.
07640 of X has a mass of 3297146 amu. Express your answer numerically. Al-28 has a much shorter half-life of 23 minutes.
Of nuclides that can be found in more than trace amounts the highest number of neutrons is 146 found in uranium-238. Which ones of the following list of molecules should have their charge written out with their name. Part B A certain element X has four isotopes.
THIS IS THE BEST ANSWER. For zinc the atomic weight is 6539 so the mass number is closest to 65. Number of Neutrons Mass Number - Number of Protons 1 - 1 0.
9493 of X has a mass of 3197207 amu. C A chemical reaction involves the rearrangement of atoms. This implies Number of neutrons 28-13.
D All atoms of a given element are identical. Finally subtract the number of protons from the rounded up atomic weight to find the number of neutrons in the atom. This element is found in group 15 and period 2 of the Periodic Table of the Elements.
Start your free trial. So weve demonstrated that for an aluminum atom which has the atomic number of 13 and has a mass number of 27 there are 13 protons 14 neutrons and 13 electrons. So there are 19 neutrons in 36CL.
Experts are tested by Chegg as specialists in their subject area. How many neutrons are found in one atom of 28Al. Question 1 Arrange the following atoms in increasing order of size from smallest to largest.
A Elements are composed of atoms. Mass number refers to the sum of protons and neutrons in the nucleus of an atom. How many protons neutrons and electrons are in a neutral atom of aluminum 28.
The amount of neutrons are found in one atom of 20f would definitely have to be 20f has around 11 neutrons. 36 17 19. Further ExplanationAn atomAn atom is the smallest particle of an element that can take part in a chemical reaction.
Round up the atomic weight to the nearest whole number. In the case of niobium 93 minus 41 is 52 which means that a niobium atom has 52 neutrons. Al-26 is an aluminum isotope with a half-life of 730000 years.
4290 of X has a mass of 3396786 amu. 13207 atomamu 8274 amu 258 atoms 3207 amuatom 5213 x. The highest number of neutrons in a stable nuclide is 126 found in lead-208.
A neutral atom of aluminum also has 13 electrons. We review their content and use your feedback to keep the quality high. Niobium has an atomic weight of 92906 so you would round it up to 93.
THIS USER ASKED How many neutrons are found in one atom of 28al. It has an atomic weight of 14007 amu. Some atoms of aluminum do not have 14 neutrons.
Therefore if you subtract the atomic number from the atomic mass you end up with the number of neutrons in the nucleus. You can write the symbol for the isotope chlorine-36 in nuclear notation also called isotopic notation as follows.

Solved 3 Part A How Many Neutrons Are Found In One Atom Of Chegg Com
Solved How Many Neutrons Are Found In One Atom Of 16n Chegg Com
Solved 3 Part A How Many Neutrons Are Found In One Atom Of Chegg Com
Comments
Post a Comment